Draw the Newman projection of the most stable conformation of 3,3-dimethylhexane viewed through the C3—C4 bond
- Mayya Alocci
- Jun 18
- 1 min read
First, we need to draw 3,3-dimethylhexane.
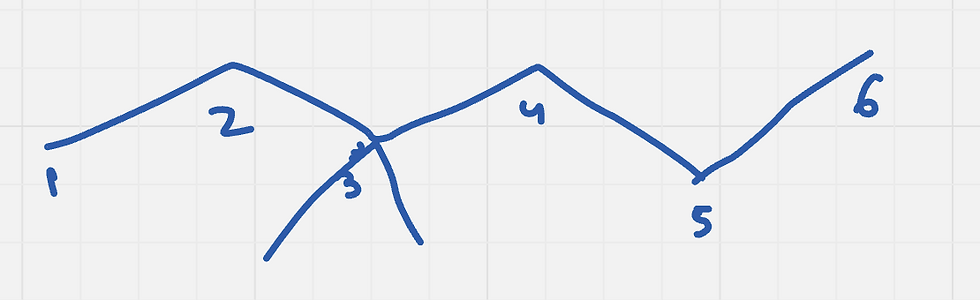
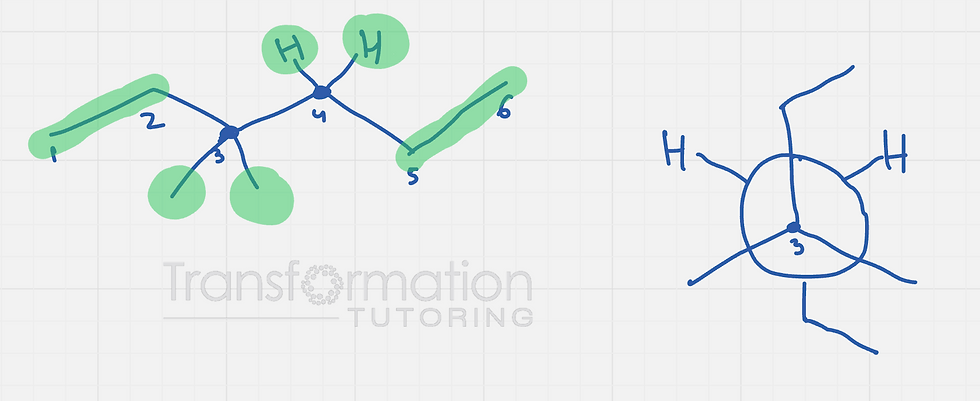
Next, we need to look at carbon 3 and find all the groups it is attached to other than carbon 4 . Carbon 3 is attached to two methyl groups and an ehtyl group. Carbon 4 is attached to two hydrogens, and one ethyl group.
The most stable conformation is staggered where the biggest groups on the two carbons are as far apart as possible .
LINKS:

Comments