Some information about the two naturally occurring isotopes of gallium is given in the table below.
- Aug 10, 2023
- 1 min read
June 2017 NY Regents Chemistry Exam
32 Some information about the two naturally occurring isotopes of gallium is given in the table below.
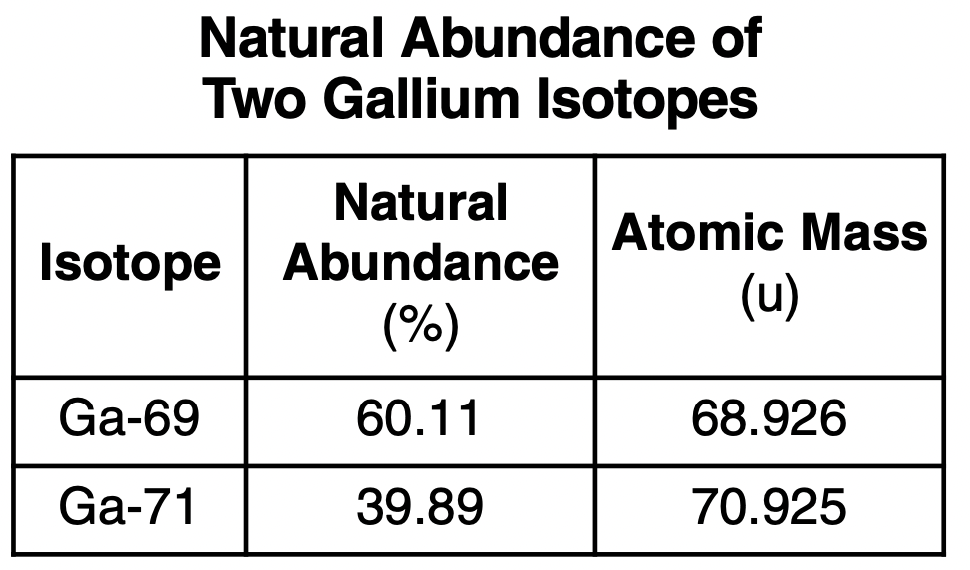
Which numerical setup can be used to calculate the atomic mass of gallium? (1) (0.6011)(68.926 u) + (0.3989)(70.925 u)
(2) (60.11)(68.926 u) + (39.89)(70.925 u)
(3) (0.6011)(70.925 u) + (0.3989)(68.926 u)
(4) (60.11)(70.925 u) + (39.89)(68.926 u)
Solution: To calculate the average atomic mass of an element, we take the atomic mass of each isotope and multiply by percent abundance in the decimal form ( divide by 100).
Answer: 1
Prepare for the Chemistry Regents Exam: HERE
Contact us for Regents Chemistry Tutoring: HERE

Comments