top of page
Expert Chemistry, Biology, Physics and Math Tutoring Online and Bethesda,MD
646-407-9078
Search
Identify the element that contains the following number of protons and neutrons: 9 protons and 9 neutrons.
Identify the element that contains the following number of protons and neutrons: 9 protons and 9 neutrons. A)F B)N C)O D)C Solution: Atom...
How many grams of sulfur (S) will be required to react with 5 g of sodium (Na) to produce Na2S
How many grams of sulfur (S) will be required to react with 5 g of sodium (Na) to produce Na2S? A) 3.5 B) 7.0 C) 1.7 D) 6.4 Solution:...
Which of the following pairs of elements would be most likely to share similar properties?
Which of the following pairs of elements would be most likely to share similar properties? A) N and O B) B and Si C) Na and Mg D) Br and...
Write the formula for the ionic compound formed from magnesium and sulfur.
Write the formula for the ionic compound formed from magnesium and sulfur. A) MgS B) MgS2 C) Mg2S D) MgS3 E) Mg3S2 Solution: Writing...
Which of the following liquids, at the same temperature, has the lowest vapor pressure?
Which of the following liquids, at the same temperature, has the lowest vapor pressure? A) CH3-O-CH3 B) CH3-CH2-CH2-CH3 C) CH3-CH2-CH3 D)...

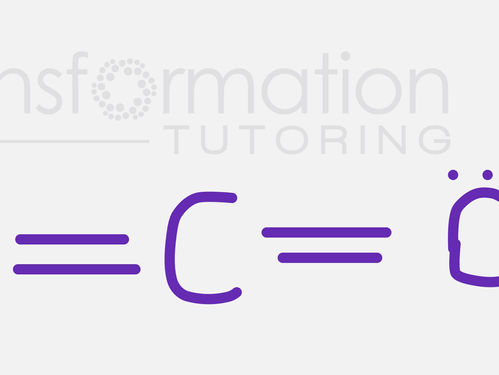
How many σ-bonds and π-bonds are in a CO2 molecule?
How many σ-bonds and π-bonds are in a CO2 molecule? A) 4 σ-bonds and 0 π-bonds B) 2 σ-bonds and 2 π-bonds C) 2 σ-bonds and 4 π-bonds D) 1...
Two different values, less than 180°, are observed for the bond angles in which of the following basic structures for molecules and ions?
Two different values, less than 180°, are observed for the bond angles in which of the following basic structures for molecules and ions?...
A gas sample containing 0.3525 moles of a compound is trapped in a 2.641 liter vessel at a temperature of 28.4 °C. What is the pressure in the vessel if the gas behaves as an ideal gas?
A gas sample containing 0.3525 moles of a compound is trapped in a 2.641 liter vessel at a temperature of 28.4 °C. What is the pressure...
A 113.25 gram sample of gold is initially at 100.0 °C. It gains 20.00 J of heat from its surroundings. What is its final temperature? (specific heat of gold = 0.129 J g−1 °C−1)
The equation we need that involves heat and change in temperature is q=mcΔT where q is heat in Joules, m is mass in grams, c is specific...


Complete Guide: How to Determine Rate Law and Rate Law Constant Using Experimental Data?
Rate Law is an equation that shows how the rate of the reaction is affected by the concentration of reactants Overall order of the...
Looking for Chemistry Tutoring?
I tutor all levels of chemistry including general and organic chemistry.
bottom of page
