top of page
Expert Chemistry, Biology, Physics and Math Tutoring Online and Bethesda,MD
646-407-9078
Search


How many electrons are in the valence shell of each atom?
How many electrons are in the valence shell of each atom? (valence electrons) (a) Carbon (b) Nitrogen (c) Chlorine (d) Aluminum Are you...


Identify the atom that has each ground-state electron configuration
Identify the atom that has each ground-state electron configuration. (a) 1s2 2s2 2p6 3s2 3p4 (b) 1s2 2s2 2p4 In this video we learn 2...


Which of the following orbitals can not exist?
Which of the following orbitals can not exist: A. 2p B. 3d C. 5d D. 3f E. 160p The first quantum number is n, principle energy level, and...
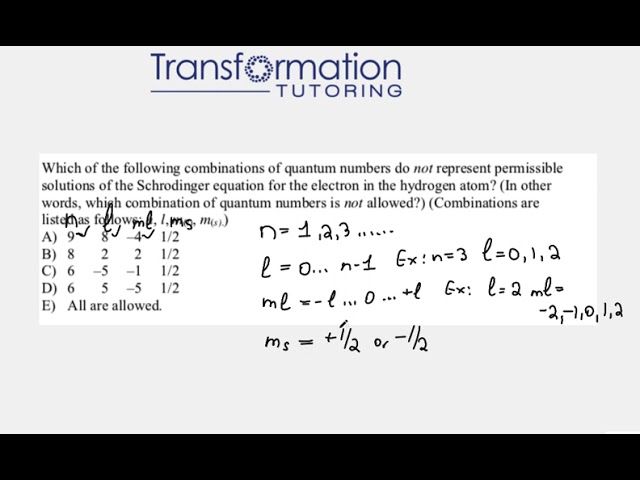

Which combination of quantum numbers is not allowed?
Which of the following combinations of quantum numbers do not represent permissible solutions of the Schrodinger equation for the...


How To Identify Bronsted- Lowry Acid Base Pairs In A Reaction
Bronsted Lowry acid is a proton (H+) donor, while the base is proton (H+) acceptor. In other words, Bronsted Lowry acid loses a...


How To Predict Shape/Molecular Geometry Of A Molecule
We will use the chart below to predict the correct shape of a molecule. First we must start with a correct Lewis dot structure of the...
How to recognize intermolecular forces?
Intermolecular forces are forces of interaction between molecules. These forces influence properties of substances such as boiling and...
What is the chemical name of the compound NH4SCN?
What is the chemical name of the compound NH4SCN? (1) ammonium thiocyanate (2) ammonium cyanide (3) nitrogen hydrogen cyanide (4)...


How to convert grams to moles?
To convert grams to moles and vice versa, we must always use Molar Mass. Molar Mass can be calculated using the Periodic Table. To...


Calculate Ka of a 0.10 M solution of lactic acid has a pH of 2.44
Lactic acid has one acidic hydrogen. A 0.1 M solution of lactic acid has a pH of 2.44. Calculate Ka for lactic acid? When we do acid/base...
Looking for Chemistry Tutoring?
I tutor all levels of chemistry including general and organic chemistry.
bottom of page
