top of page
Expert Chemistry, Biology, Physics and Math Tutoring Online and Bethesda,MD
646-407-9078
Search


How to convert grams to moles?
To convert grams to moles and vice versa, we must always use Molar Mass. Molar Mass can be calculated using the Periodic Table. To...


Calculate Ka of a 0.10 M solution of lactic acid has a pH of 2.44
Lactic acid has one acidic hydrogen. A 0.1 M solution of lactic acid has a pH of 2.44. Calculate Ka for lactic acid? When we do acid/base...
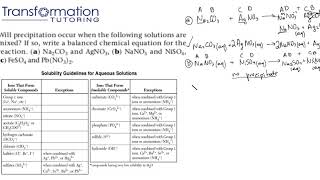

Will precipitation occur when the following solutions are mixed?
Will precipitation occur when the following solutions are mixed? If so, write a balanced chemical equation for the reaction. (a) Na2CO3...


How To Name Acids
How To Name Acids An acid is a substance that releases H+ ions (hydrogen ions) when it is dissolved in water. Acids can be divided into 2...


How to identify a weak acid?
How to identify a weak acid? A weak acid is an acid that does not dissociate completely. If we are given a Ka for an acid that is small,...


How do we know if the reaction is spontaneous?
What does it mean for a reaction to be spontaneous? It means that the reaction will occur on its own, without any outside energy input...
How to find out coordination number?
Many students in their general chemistry classes learn about coordination complexes. Coordination complexes are created when transition...


A piece of silver metal weighting 194.3g is placed in a graduated cylinder containing
First we must write the formula for density. Density = mass/volume
Now, let's figure out if we have all of the components for our equati
Looking for Chemistry Tutoring?
I tutor all levels of chemistry including general and organic chemistry.
bottom of page
