top of page
Expert Chemistry, Biology, Physics and Math Tutoring Online and Bethesda,MD
646-407-9078
Search


How is Molarity different from Molality?
Both molarity and molality are measures of concentration, or how much solute is dissolved in solvent. Let's first get acquainted with a...
Which electron shell in an atom of calcium in the ground state has an electron with the greatest
The further an electron is from the nucleus, the higher it's energy is. Therefore, electrons in the valence shell (outermost shell) have the
The only two elements in alkenes and alkynes are
The only two elements in alkenes and alkynes are (1) carbon and nitrogen (2) carbon and hydrogen (3) oxygen and nitrogen (4) oxygen and...


How to best prepare for chemistry finals?
It's almost december and that means it's finals time! This can be very overwhelming and stressful. At Transformation Tutoring, we came up...
What is the chemical name of the compound NH4SCN?
What is the chemical name of the compound NH4SCN? (1) ammonium thiocyanate (2) ammonium cyanide (3) nitrogen hydrogen cyanide (4)...


How to convert grams to moles?
To convert grams to moles and vice versa, we must always use Molar Mass. Molar Mass can be calculated using the Periodic Table. To...
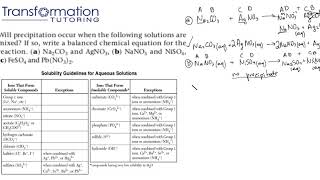

Will precipitation occur when the following solutions are mixed?
Will precipitation occur when the following solutions are mixed? If so, write a balanced chemical equation for the reaction. (a) Na2CO3...
Which notations represent atoms that have the same number of protons but a different number of neutr
Which notations represent atoms that have the same number of protons but a different number of neutrons? (1) H-3 and He-3 (2) S-32 and...


A sample of the male sex hormone testosterone,C19H28O2, contains 3.88 * 10^21 hydrogen atoms.
A sample of the male sex hormone testosterone,C19H28O2, contains 3.88 * 10^21 hydrogen atoms. (a) How many atoms of carbon does it...


How To Name Acids
How To Name Acids An acid is a substance that releases H+ ions (hydrogen ions) when it is dissolved in water. Acids can be divided into 2...
Looking for Chemistry Tutoring?
I tutor all levels of chemistry including general and organic chemistry.
bottom of page
